
This innovative offering will dramatically reduce your time to market while ensuring your device packaging will protect your product and comply with international standards and requirements.
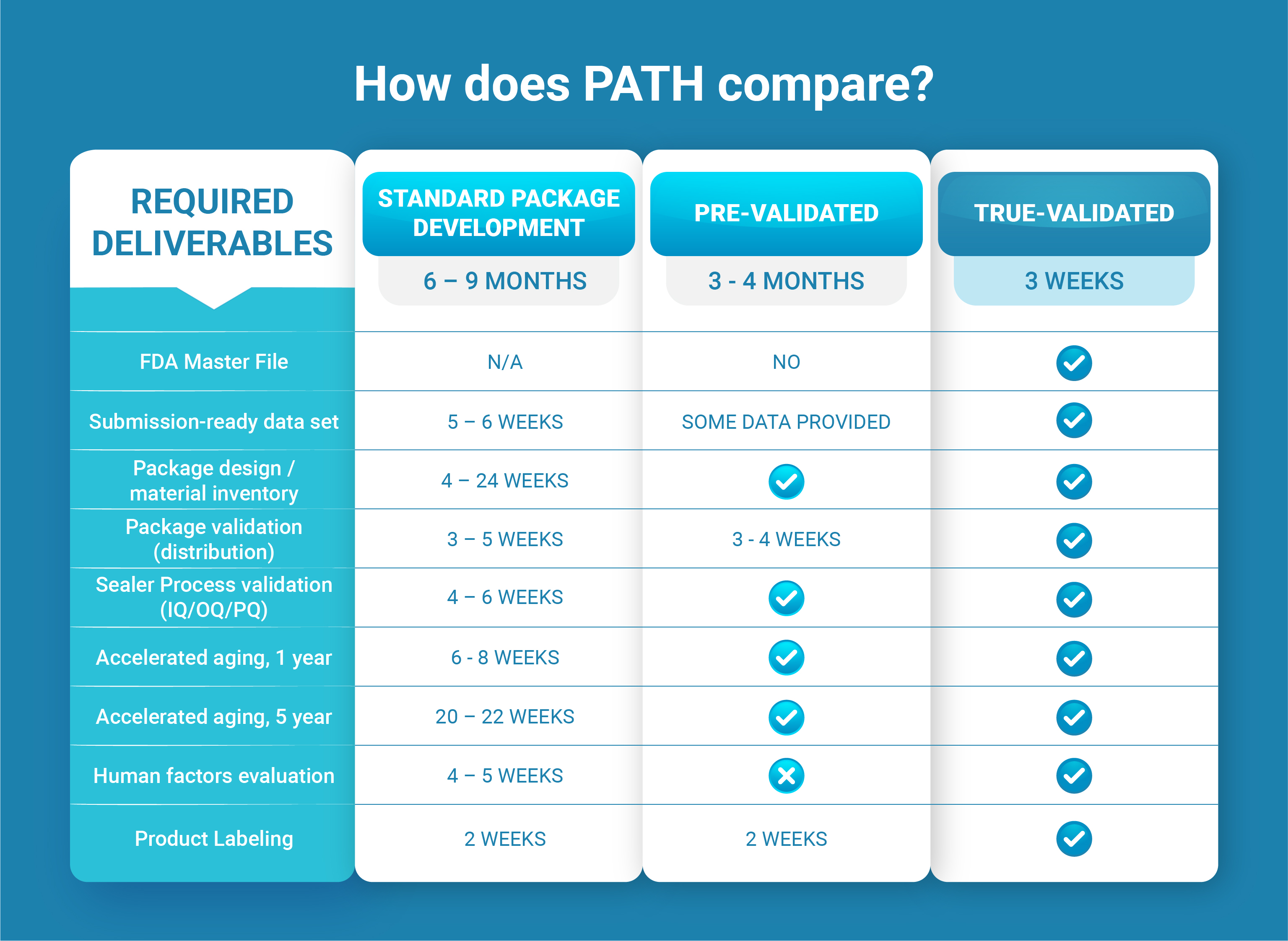
Find out how PATH™ eliminates the need to source materials, design/build/test packaging, and validate processes.
